Molnupiravir Merck & Co
Emergency use authorization for its experimental COVID-19 antiviral treatment molnupiravir before year-end. This decision follows Merck.
In July Merck entered into a molnupiravir supply pact with the US.

Molnupiravir merck & co. MRK stock closed up 241 at 7509 on Wednesday. Drugmaker Merck Co Inc said on Monday it sees potential US. It operates through two segments Pharmaceutical and Animal Health segments.
The US government has already placed an order with Merck Co to buy 17 million courses of its antiviral therapy molnupiravir for COVID-19 assuming it gets approved for use by the FDA and Merck. Merck headquarters in Kenilworth New Jersey. Reuters -Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus including the dominant highly transmissible Delta the company said on Wednesday.
Merck Sharp Dohme Corp a subsidiary of Merck Co Inc. Merck MRK and its partner Ridgeback Biotherapeutics announced the initiation of Phase 3 MOVe-AHEAD clinical trial designed to evaluate the oral antiviral molnupiravir in the. Drugmaker Merck Co Inc said on Monday it sees potential US.
Government that implies a price of 700 for a course of treatment with its antiviral molnupiravir. Government on this new agreement that will provide Americans with COVID-19 access to molnupiravir an investigational oral therapy being studied for outpatient use early in the course of disease if it is authorized or approved said Rob Davis president Merck. Emergency use authorization for its experimental COVID-19 antiviral treatment molnupiravir before year-end.
Merck and its Miami-based partner Ridgeback Biotherapeutics have started the third phase of its MOVEe-AHEAD clinical trial for molnupiravir an investigational oral. I would just say that our program is enrolling well and we expect to be able to see clinical data in the back half of the year Mercks Chief Executive Officer Robert. Photo by Arek Socha from Pixabay.
MRK known as MSD outside the United States and Canada today announced that the company is discontinuing development of its SARS-CoV-2COVID-19 vaccine candidates V590 and V591 and plans to focus its SARS-CoV-2COVID-19 research strategy and production capabilities on advancing two therapeutic candidates MK-4482 and MK-7110. Merck has a contract with the US. Shares of established health care company Merck Co Inc.
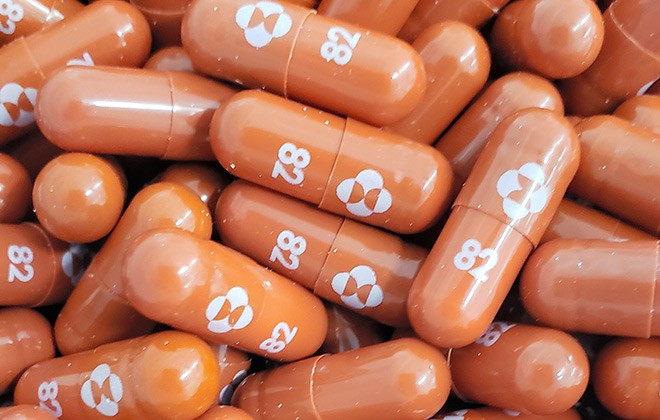
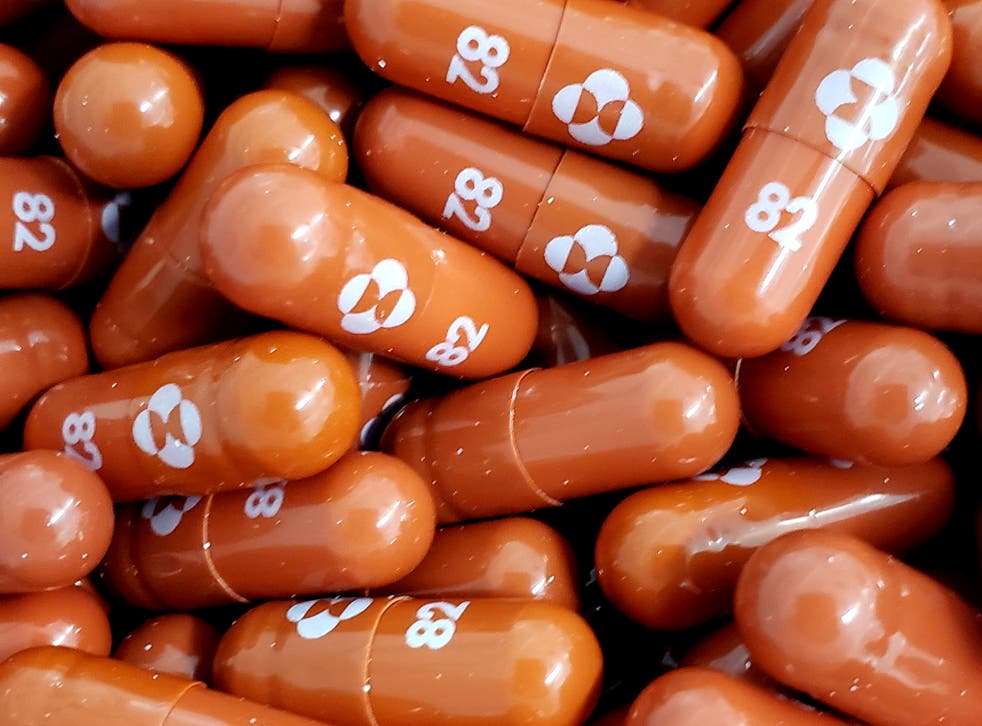
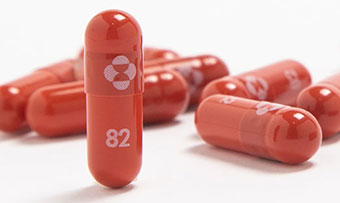
An experimental COVID-19 treatment pill called molnupiravir being developed by Merck Co Inc and Ridgeback Biotherapeutics LP is seen in this undated handout photo released by Merck. MRK which is headquartered in Kenilworth NJ have lost 84 over the past month to close yesterdays trading session at 7204 due mainly to investor pessimism around the company not being a frontrunner in the COVID-19 vaccine raceHedge funds interest in the name has remained unchanged from the last. Since molnupiravir does not target the spike protein of the virus - the target of all current COVID-19 vaccines - which defines the.
Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus including the dominant. Operates as a healthcare company worldwide. The Pharmaceutical segment offers human health pharmaceutical products in the areas of oncology hospital acute care immunology neuroscience virology cardiovascular diabetes and womens health as well as vaccine products.
Merck is pleased to collaborate with the US. Merck revealed a deal with the United States to supply 17 million courses of experimental COVID-19 oral treatment molnupiravir for 12 billion. Merck Co Inc.
Merck called MSD outside the US and Canada has initiated a rolling submission to Health Canada for molnupiravir an investigational oral antiviral therapy for the treatment of Covid-19. An effective convenient COVID-19 treatment could reach annual sales of over 10 billion according to a recent Jefferies Co estimate.

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg

Three Pharma Cos In Race To Find Covid 19 Treatment Pill Results Likely By 2021 End Businesstoday

Merck And Ridgeback S Investigational Oral Antiviral Molnupiravir Reduced The Risk Of Hospitalization Or Death By Approximately 50 Percent Compared To Placebo For Patients With Mild Or Moderate Covid 19 In Positive Interim Analysis

Covid Pill Works Against All Variants Including Delta Manufacturer Says The Independent